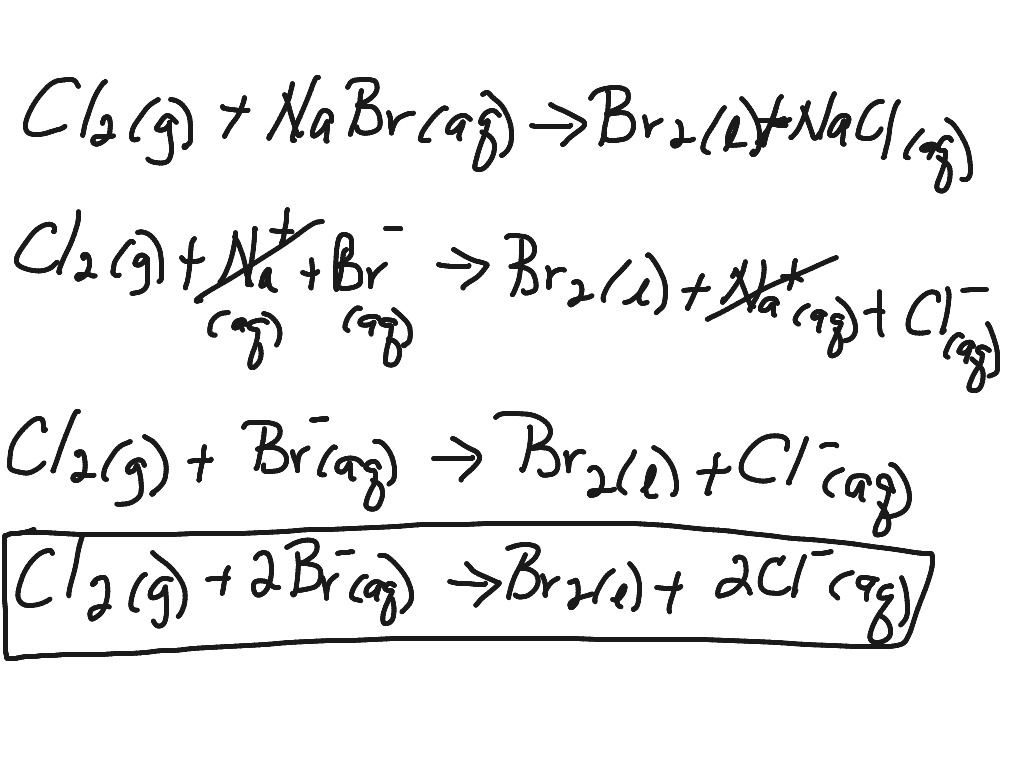Wonderful Info About How To Write An Ionic Equation

Pptx, 1.63 mb.
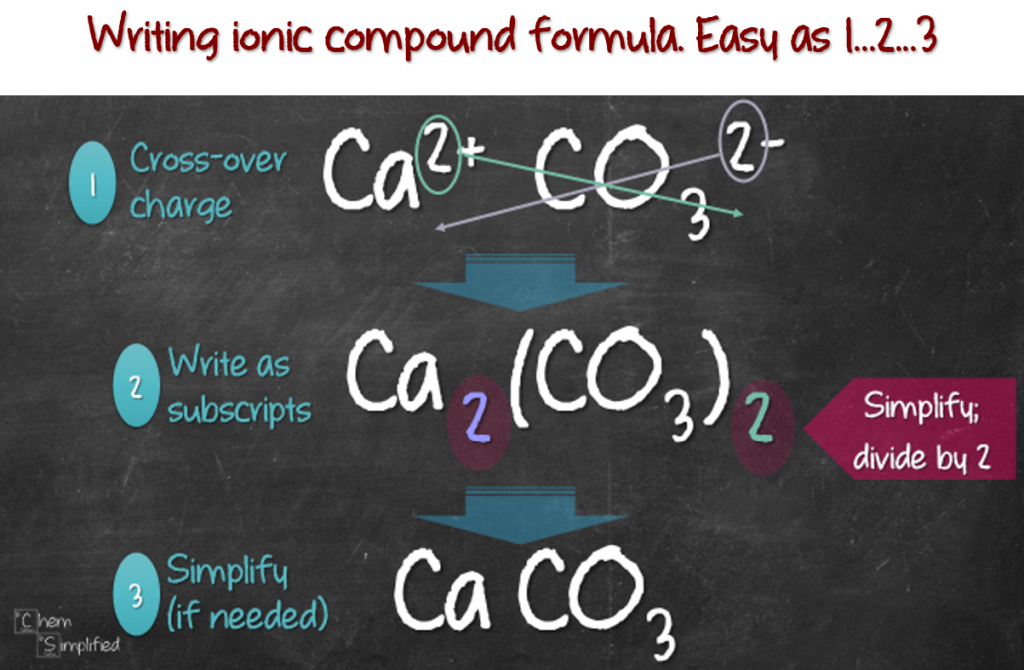
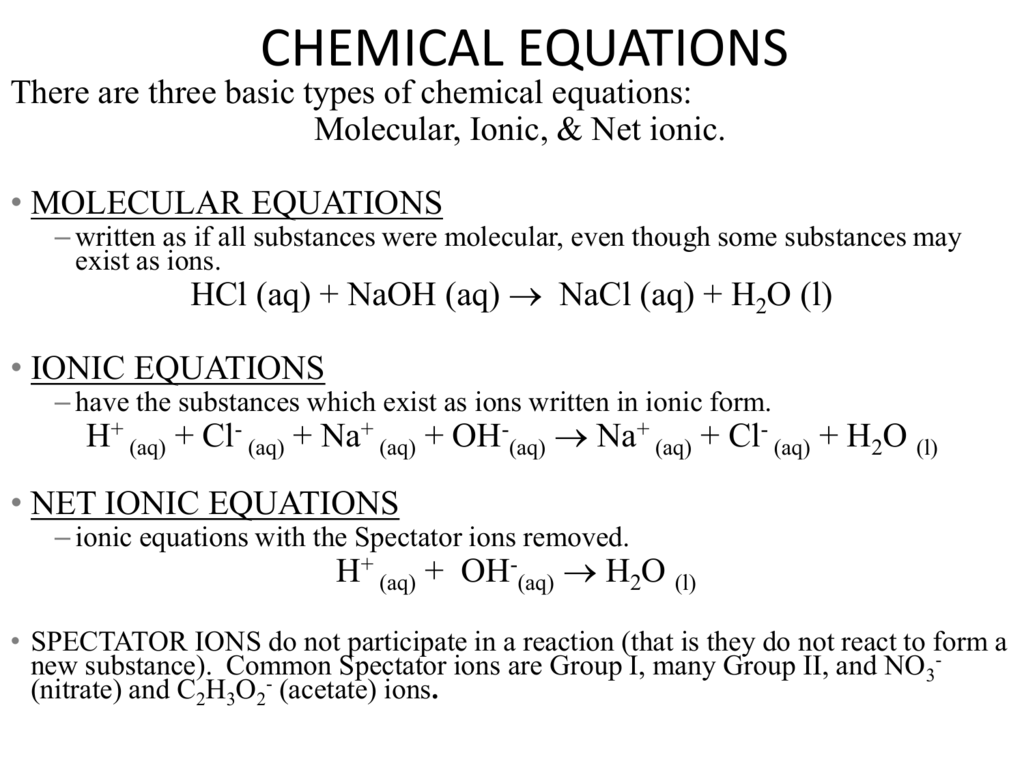
How to write an ionic equation. N a+ + so2− 4. Ionic compounds do not exist as molecules. What you want to do is make the compound neutral.
Total charges carried by the ions must be balanced (e.g. Calculate net ionic equation. These equations can be used to represent what happens in.
Enter an equation of an ionic chemical equation and press the balance button. Number of atoms of each elements must be balanced. The balanced equation will be calculated along.
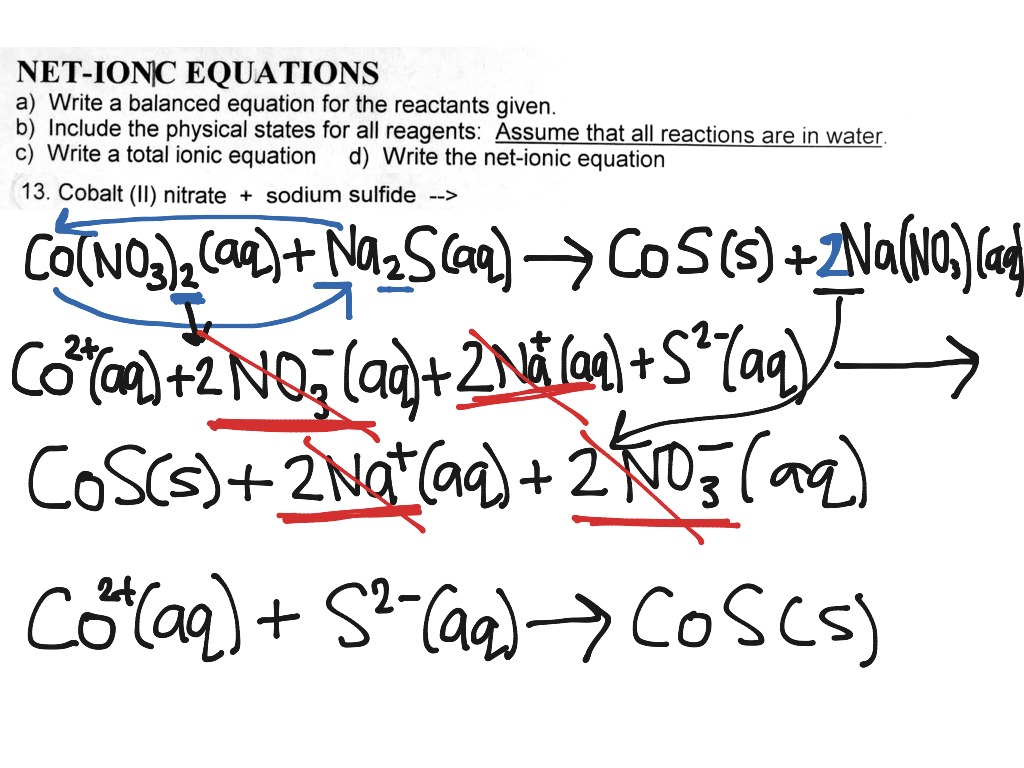
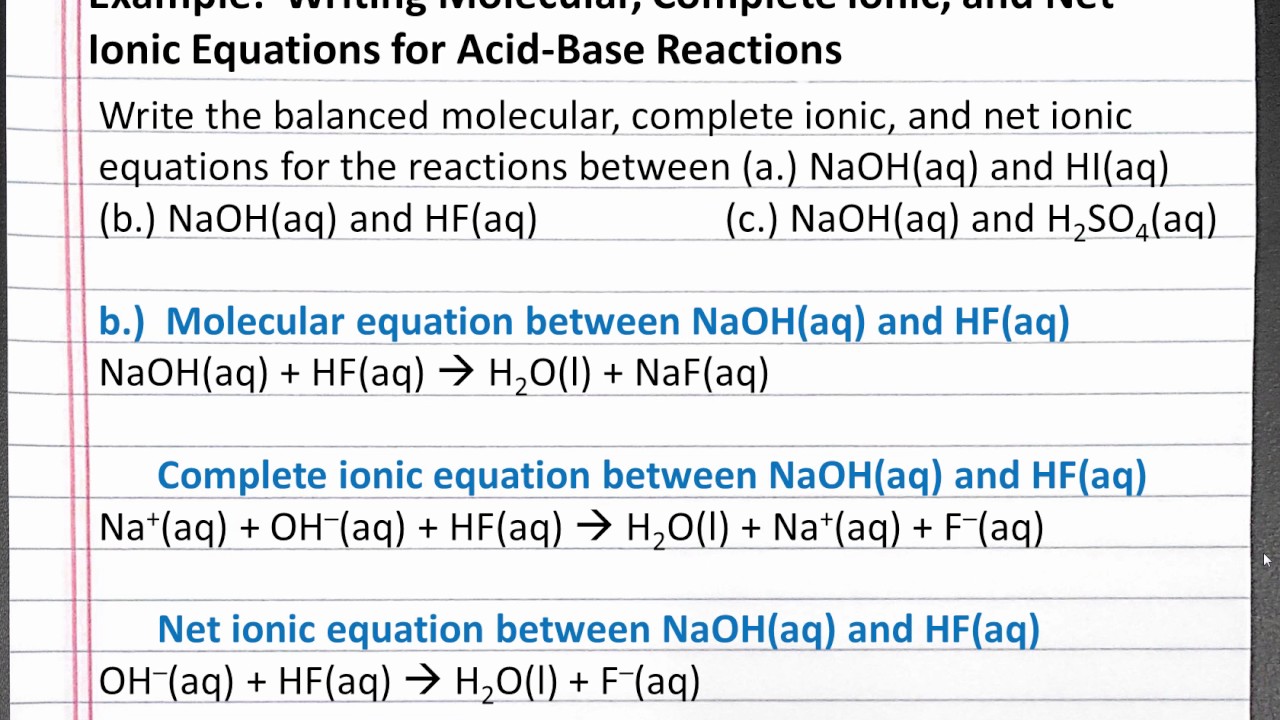
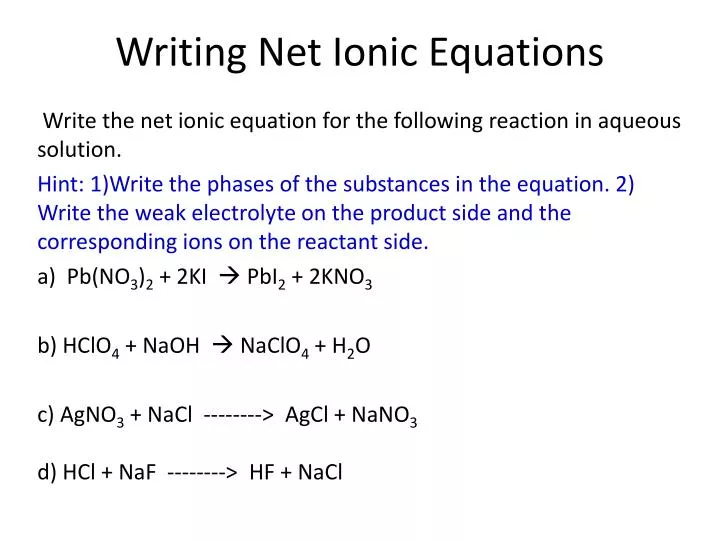
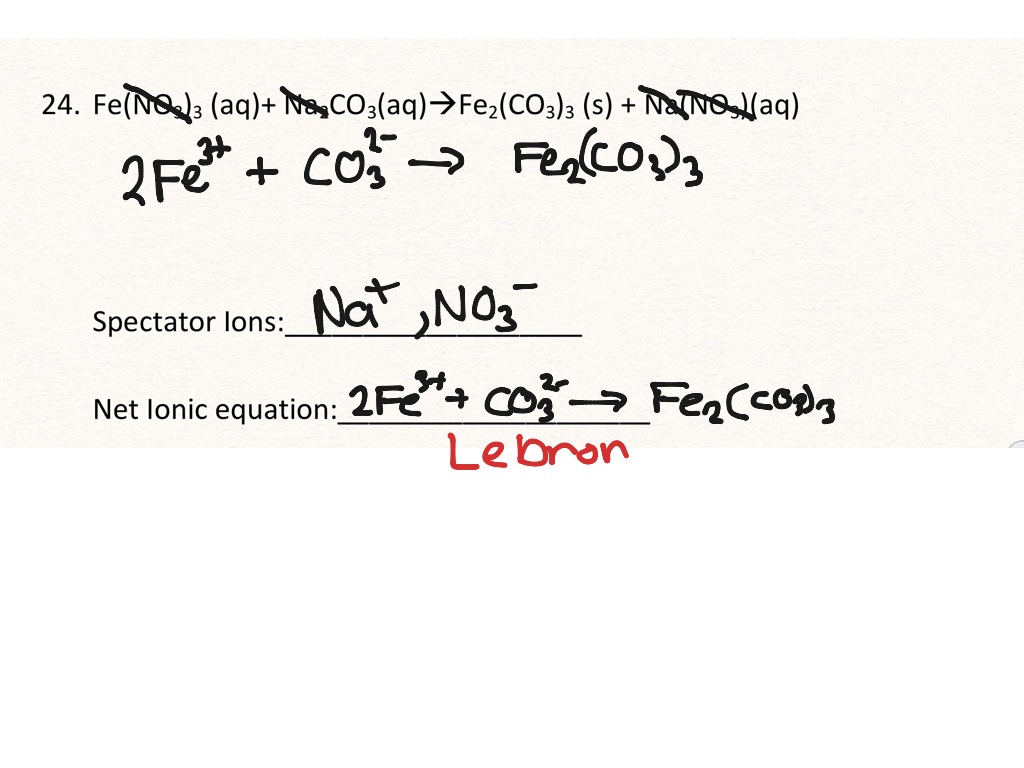
Learn how to write ionic equations for any reaction involving aqueous substances (ions in a solution). Let's take the following example: Complete and net ionic equations.
When chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the complete ionic formula. Writing ionic equations for redox reactions. Recognize polyatomic ions in chemical formulas.
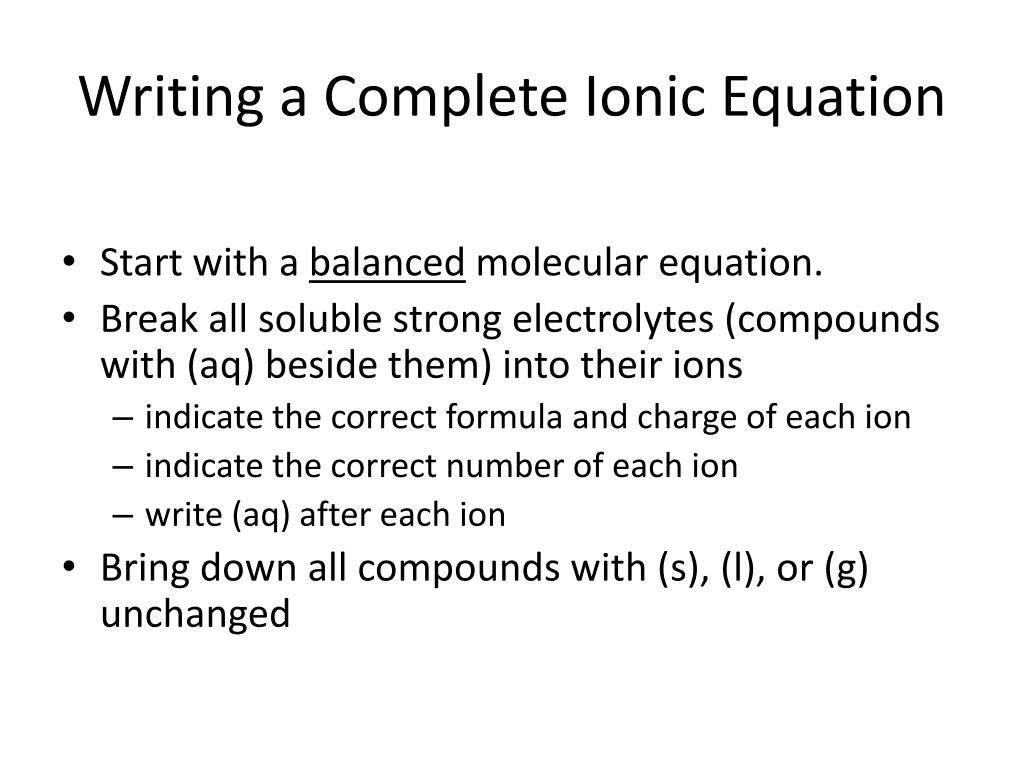
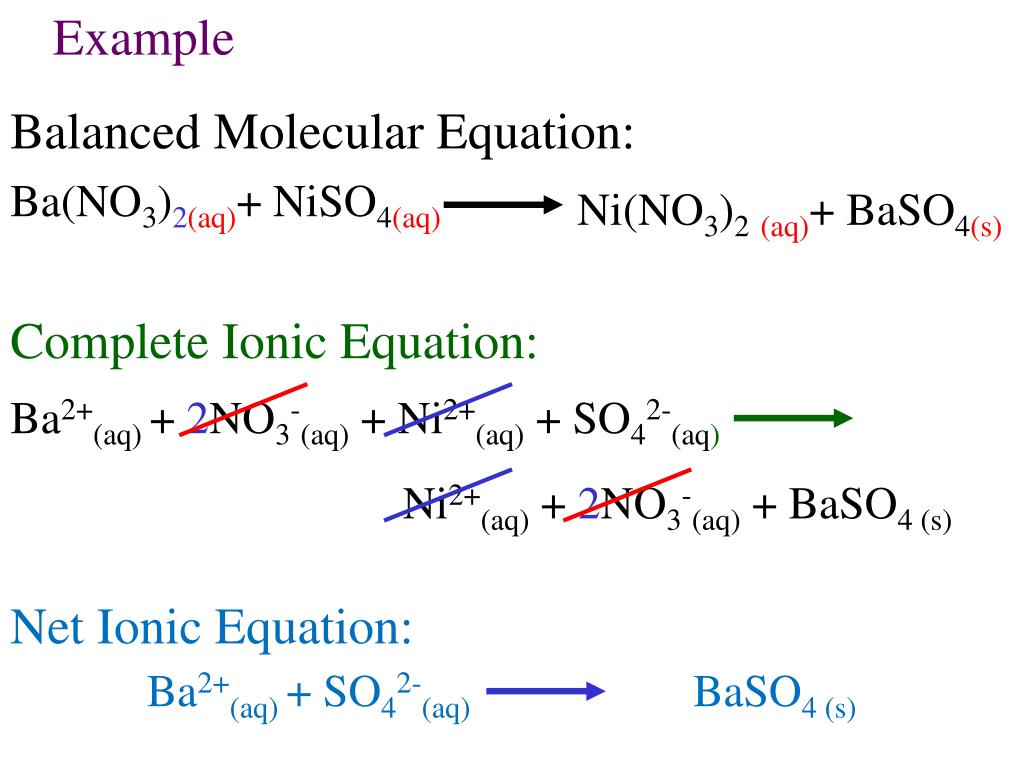
There are three steps to writing a net ionic equation: In the molecular equation for a reaction, all of the reactants and products are represented as neutral molecules (even soluble ionic compounds and strong acids). +3 on left must have +3 on right as well) spectator.
Balancing the molecular equation, transforming to a complete ionic equation (how each species exists in solution),. There are two different types of ionic equations: Write the equation in terms of all of the.
Mainly for year 11 but could be useful for year 12. Write the correct formula for an ionic compound. A complete ionic equation is a chemical equation in which.
There are three basic steps to writing a net ionic equation: Then write the ionic equation, showing all aqueous substances as ions. , the acid’s hydrogen ions react with.
How do you write the formula of an ionic compound? A step by step guide on how to write ionic equations. It explains how to predict the products of double replacement reactions and acid base.