Lessons I Learned From Info About How To Write Polyatomic Formulas
The crisscross method is a useful technique for writing chemical formulas of compounds with polyatomic ions.
How to write polyatomic formulas. 39k views 5 years ago complete guide to naming and formula writing for chemical compounds. Writing formulas with polyatomic ions. This is a practice quiz for writing formulas for polyatomic and monatomic ions, include correct charges.
This easy to understand tutorial covers how polyatomic ions are different from regular ions. Writing formulas for ionic compounds containing polyatomic ions. The atoms in a polyatomic ion.
This chemistry video explains the process of writing chemical formulas for ionic compounds with polyatomic ions, transition metals and roman numerals. The formula writing and naming of polyatomic ionic compounds is similar to the process for binary compounds. We have already encountered some.
The rules that you have to follow while writing a chemical formula are as follows: Write the chemical formula for an ionic compound and name them. Find the name and charge of.
Write the correct formula for an ionic compound. Writing formulas for ionic compounds containing polyatomic ions involves the same steps as for a binary. The overlapping of atomic orbitals.
Writing formulas for polyatomic compounds. For co 32−, for example, we. Understand how to write chemical formulas for binary and polyatomic ionic compounds and see examples.
The use of roman numerals in chemical formulas indicates the. Below are some more examples of compounds with monoatomic ions: This chemistry video tutorial explains how to memorize the polyatomic ions.
The writing formula of compounds containing polyatomic ions is the same as writing the formula of a binary ionic compound, except that the polyatomic ions must remain intact. If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion. Ionic compounds do not exist as molecules.
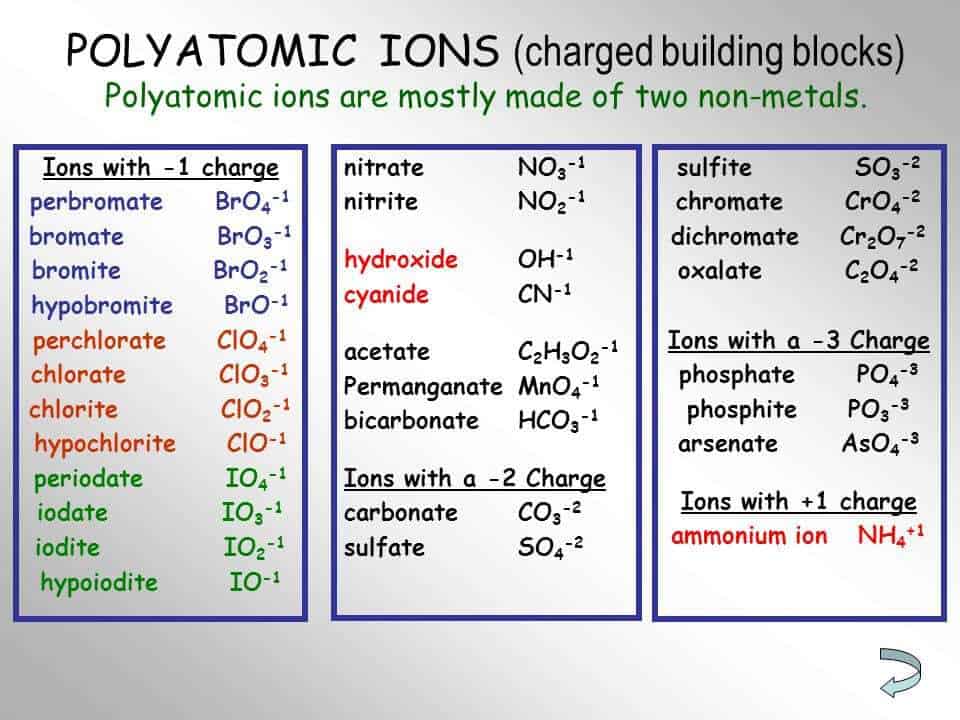
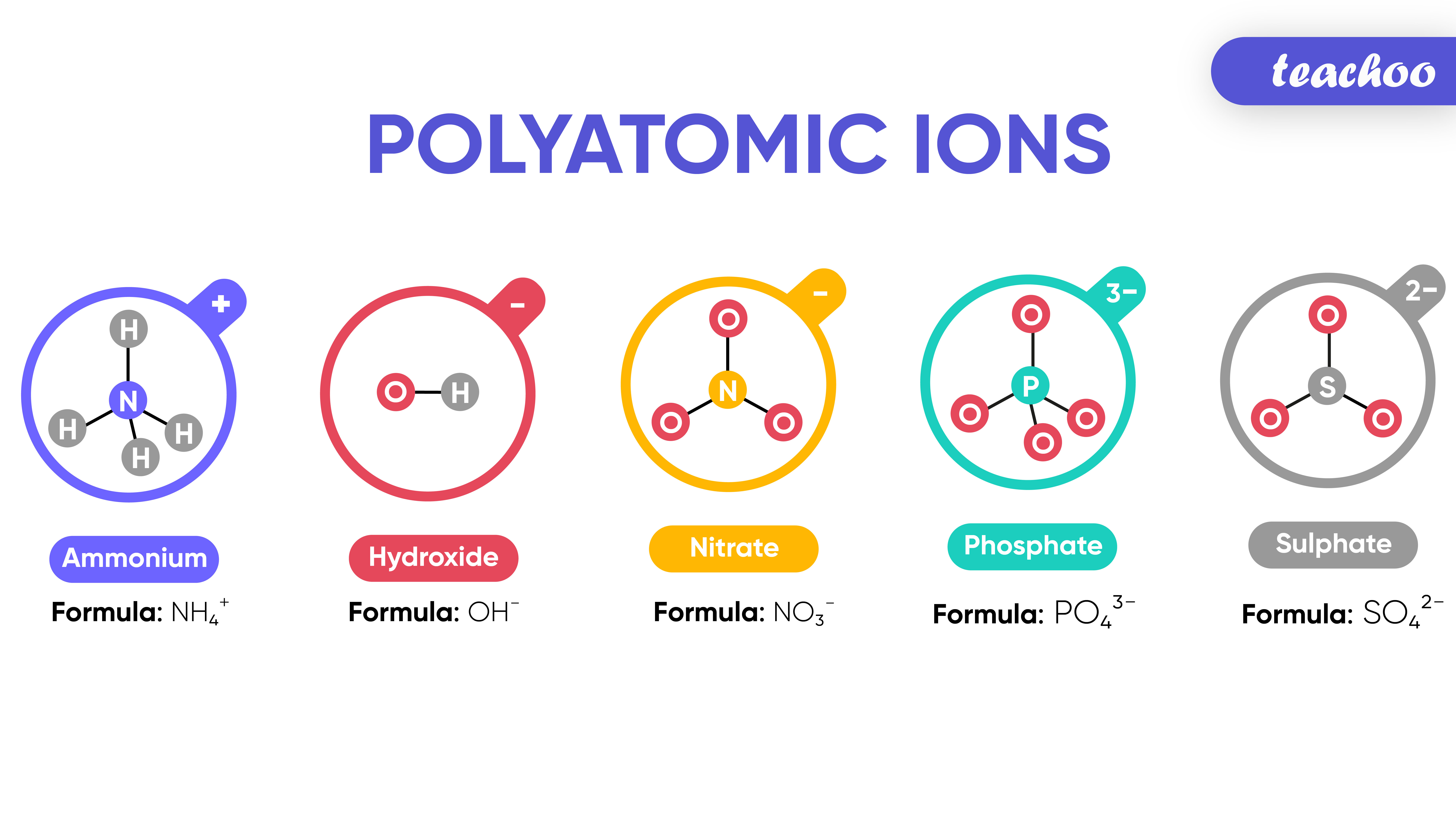
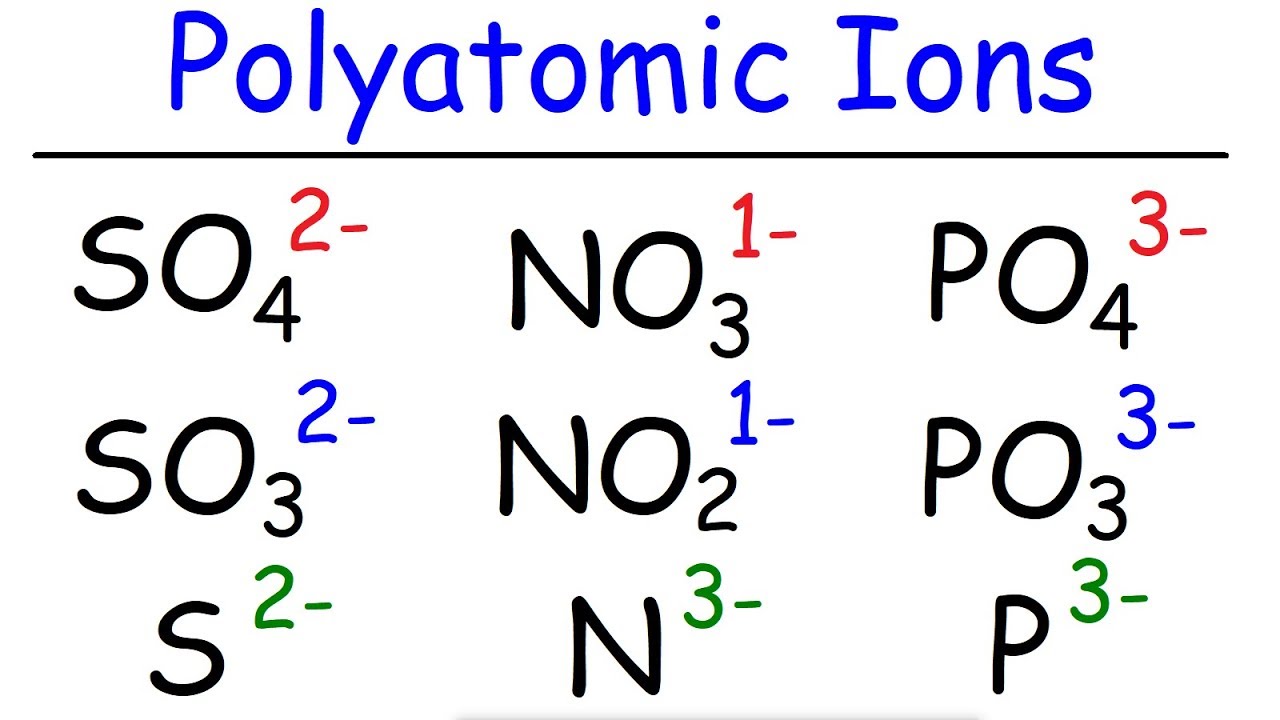
In order to write formulas for. Recognize polyatomic ions in chemical formulas. It provides the name of the common polyatomic ions, the charges and their respective formulas as well.
Recognize polyatomic ions in chemical formulas. Writing formulas with polyatomic ions. Writing formulas for ionic compounds containing polyatomic ions.